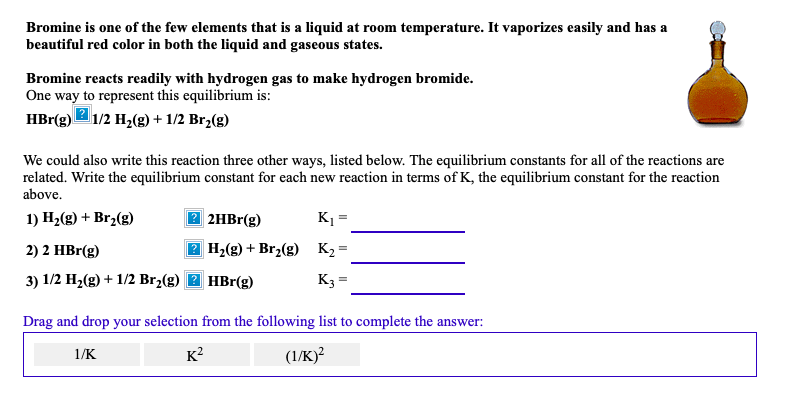
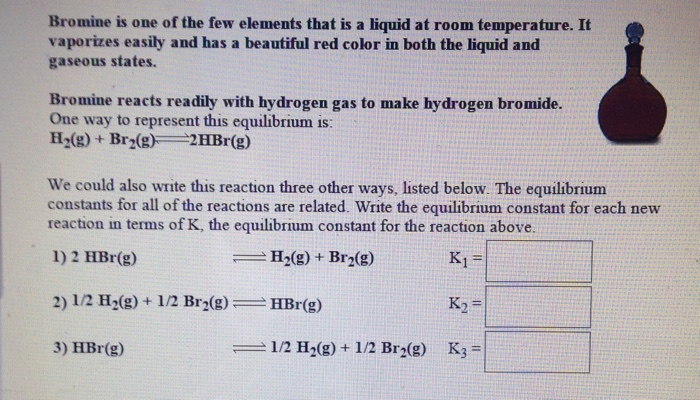
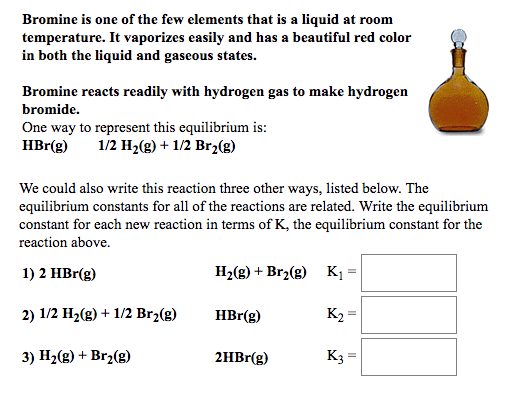
It is the third lightest halogen and is a fuming red brown liquid at room temperature that evaporates readily to form a similarly coloured gas.
Bromine element state at room temp.
Therefore both these elements have 7 electrons in their outermost electron shell.
Bromine chemical element a deep red noxious liquid and a member of the halogen elements or group 17 of the periodic table.
Sublimation the transition of a substance directly from the solid to the gas phase without passing through a liquid phase.
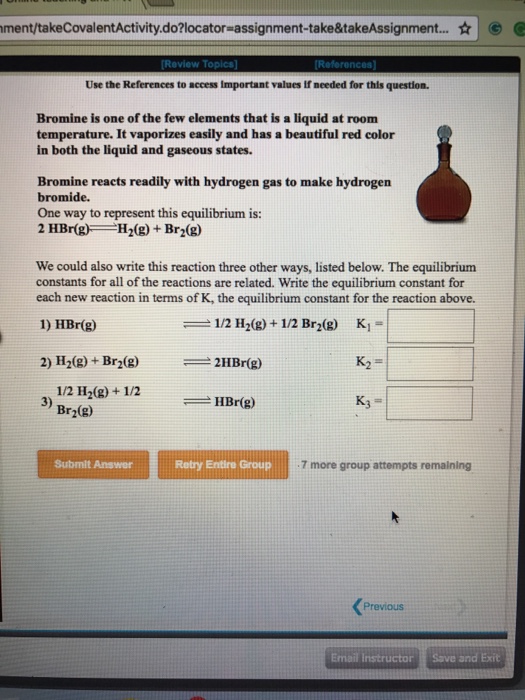
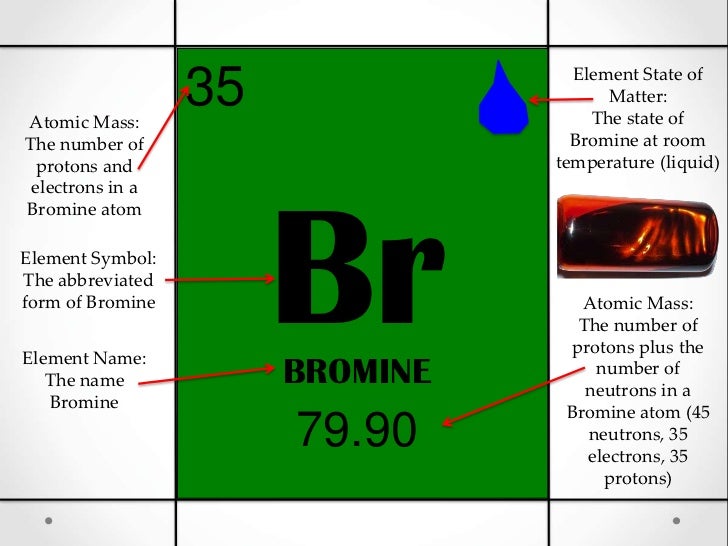
Bromine is a chemical element with the symbol br and atomic number 35.
Astatine is placed below iodine in group 7.
When he showed his professor leopold gmelin the red smelly liquid he had produced gmelin realized that this was an unknown substance and.
Gaseous liquid plasma or solid.
The only nonmetallic element that is a liquid at normal room temperatures bromine was produced by carl löwig a young chemistry student the summer before starting his freshman year at heidelberg.
Melting and boiling points of group 7 elements state at room temperature.
Jordan israel china and the united states are major producers of bromine.
While temperature is an easily controlled factor manipulating pressure is another way to cause a phase change.
Room temperature is usually taken as being 25 c.
Density g cm 3 density is the mass of a substance that would fill 1 cm 3 at room temperature.
When pressure is controlled other pure elements may be found at room temperature.
At this temperature fluorine and chlorine are gases bromine is a.
Other liquid elements.
Bromine and iodine are elements in the halide group or the group 17 of the periodic table.
Liquid which scientist described an atom made of a solid positively charge substance with electrons dispersed throughout it.
Its properties are thus intermediate between those of chlorine and iodine isolated independently by two chemists carl jacob löwig in 1825 and antoine jérôme balard in 1826.
Natural salt deposits and brines are the main sources of bromine and its compounds.
Chadwick thomson rutherford or bohr.
This is because it s boiling point is 59 degrees celsius which is 39 degrees.
That state of matter of an element may be predicted based on its phase diagram.
The graph shows the melting and boiling points of the first four group 7 elements.
Relative atomic mass the mass of an atom relative to that of.
An example is the halogen element chlorine.
Bromine element occurs in the state at room temperature.
Predict the melting and boiling points of astatine and its state at.